有机化学 ›› 2021, Vol. 41 ›› Issue (6): 2228-2248.DOI: 10.6023/cjoc202010039 上一篇 下一篇
综述与进展
底慧明( ), 刘云婷, 马艳榕, 杨鑫悦, 金辉*(
), 刘云婷, 马艳榕, 杨鑫悦, 金辉*( ), 张立新*(
), 张立新*( )
)
收稿日期:2020-10-28
修回日期:2020-11-18
发布日期:2021-02-22
通讯作者:
金辉, 张立新
基金资助:
Huiming Di( ), Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin(
), Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin( ), Lixin Zhang(
), Lixin Zhang( )
)
Received:2020-10-28
Revised:2020-11-18
Published:2021-02-22
Contact:
Hui Jin, Lixin Zhang
Supported by:文章分享

3,4-二氢吡喃-2-酮和3,4-二氢吡啶-2-酮结构广泛存在于多种天然产物和具有药理活性的分子中, 因此高效合成手性3,4-二氢吡喃-2酮和3,4-二氢吡啶-2酮受到越来越多药物化学和有机化学研究者的关注. 近年来有机小分子催化的不对称反应已成为构建手性杂环化合物的有效方法. 综述了自2003年至今有机小分子催化的不对称反应合成手性3,4-二氢吡喃酮和3,4-二氢吡啶酮衍生物的研究进展, 并根据不同的催化剂类型及反应机理进行分类讨论.
底慧明, 刘云婷, 马艳榕, 杨鑫悦, 金辉, 张立新. 有机催化不对称合成3,4-二氢吡喃-2-酮和3,4-二氢吡啶-2-酮衍生物研究进展[J]. 有机化学, 2021, 41(6): 2228-2248.
Huiming Di, Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin, Lixin Zhang. Recent Advances in Organocatalytic Asymmetric Synthesis of 3,4-Dihydropyran-2-ones and 3,4-Dihydropyridin-2-ones[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2228-2248.

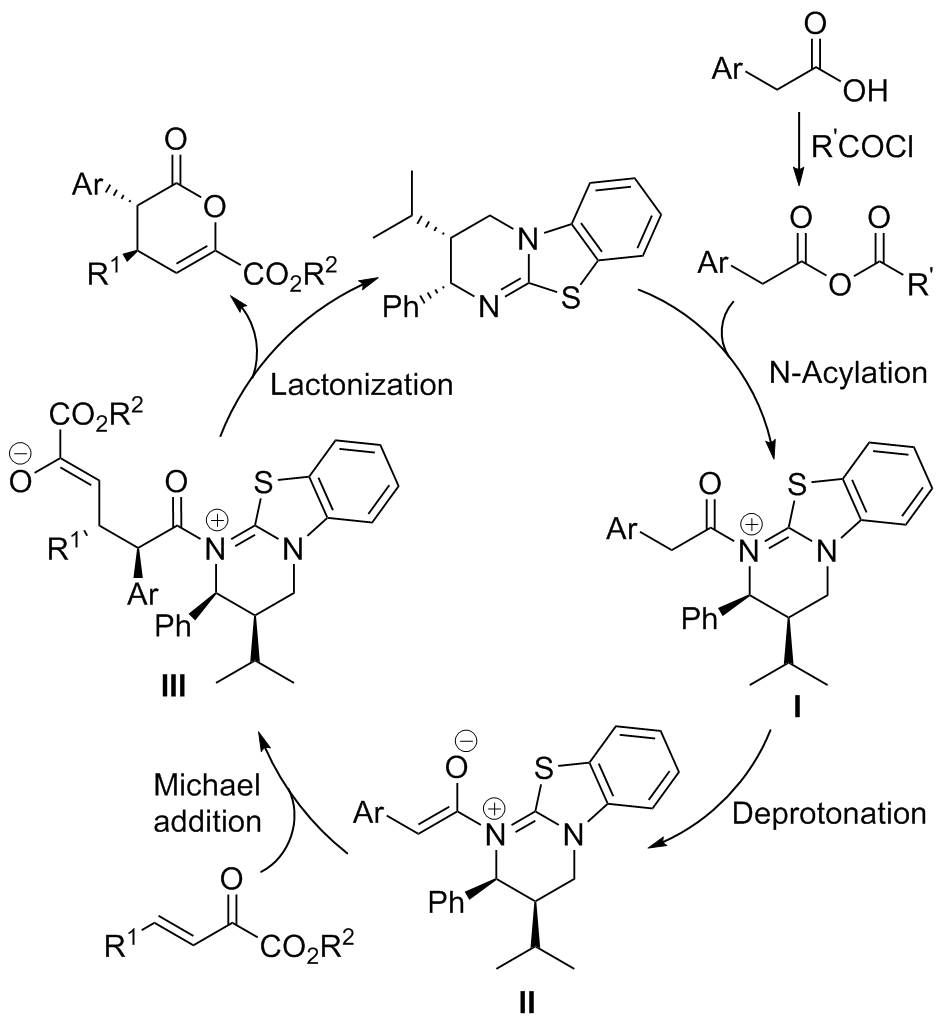

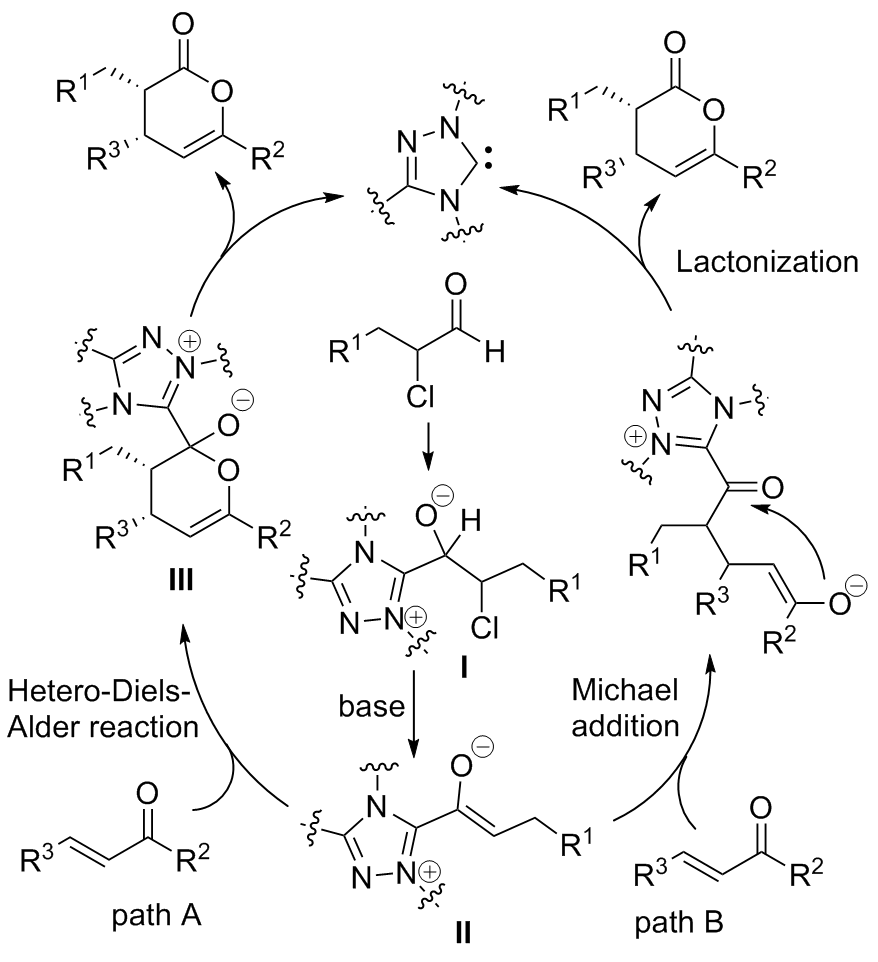

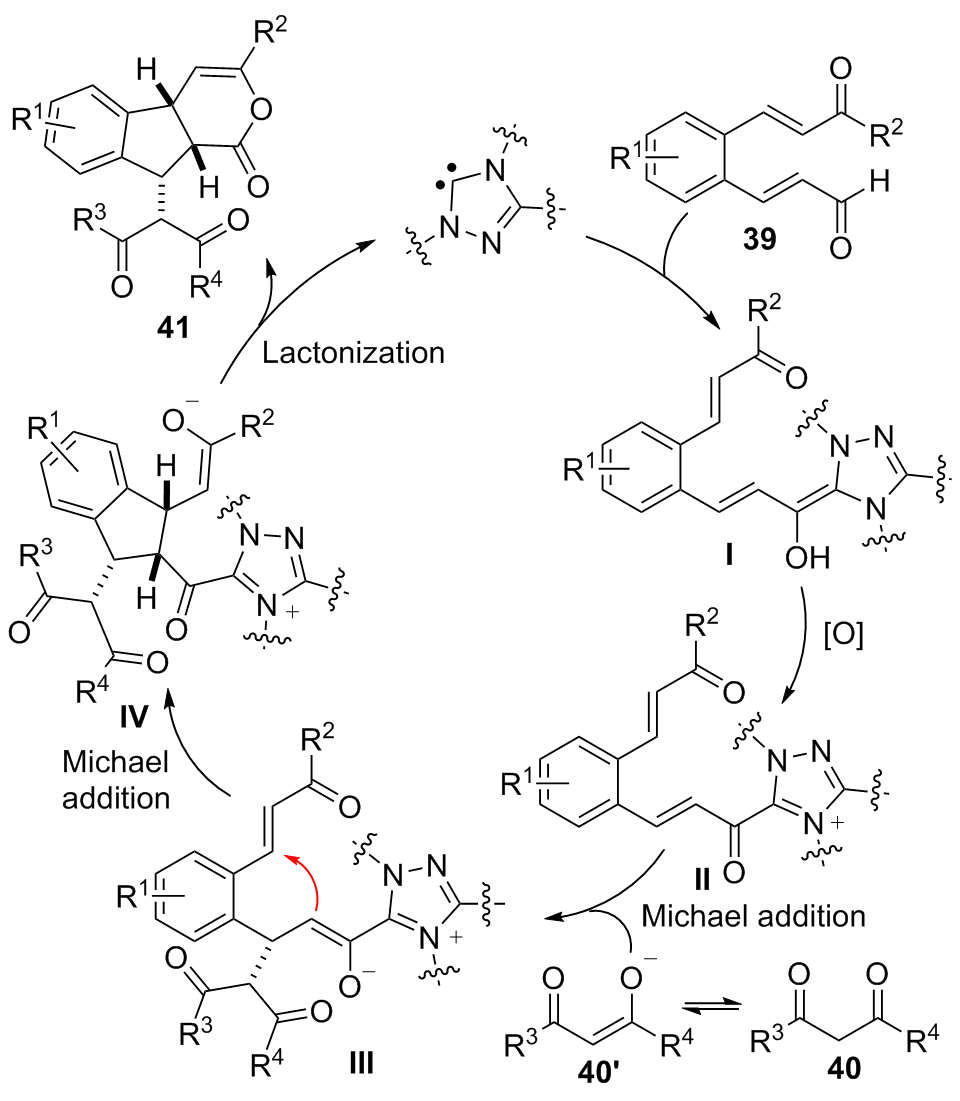






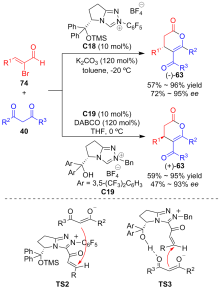
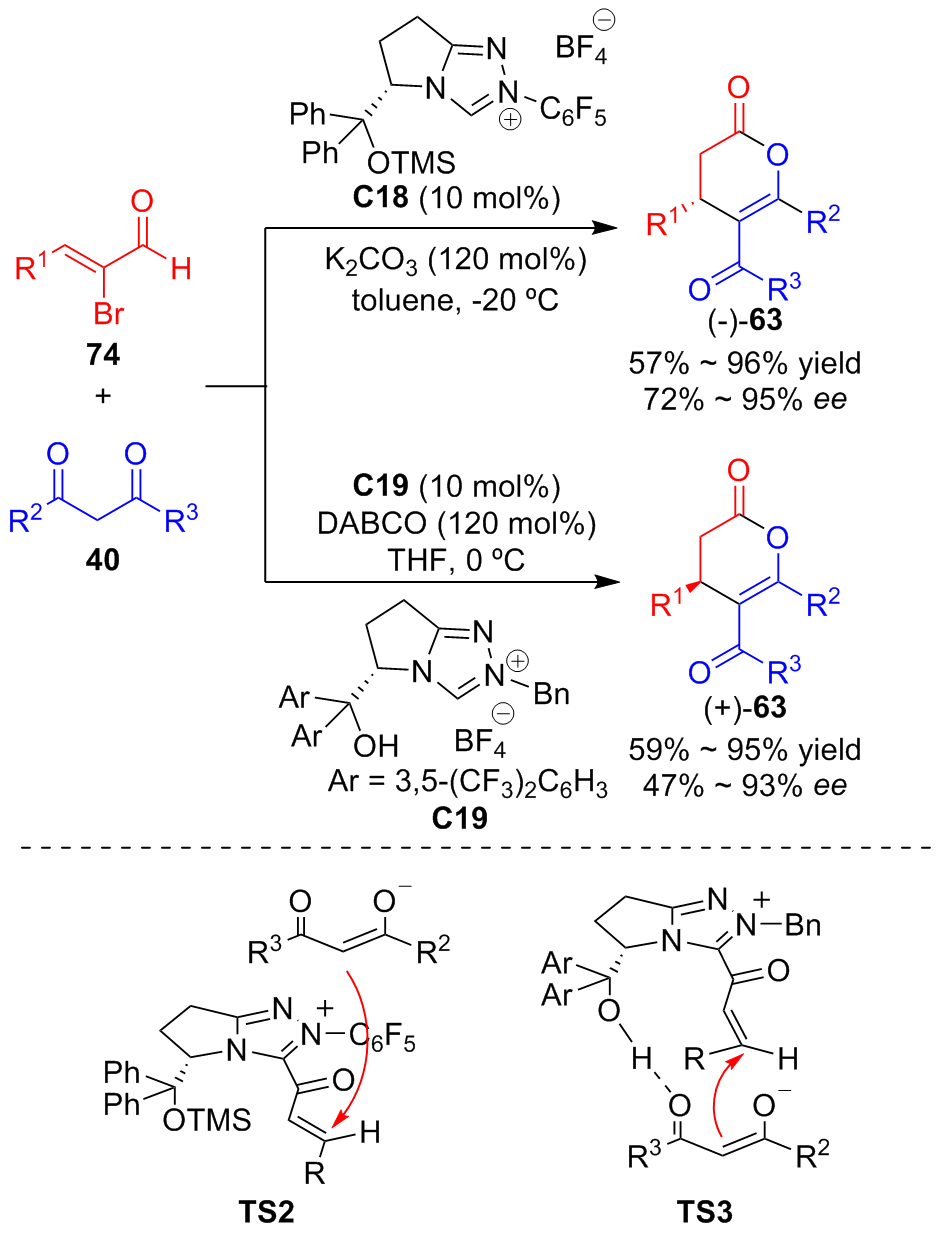
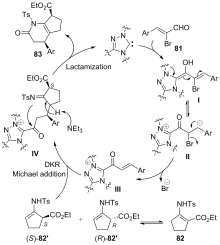








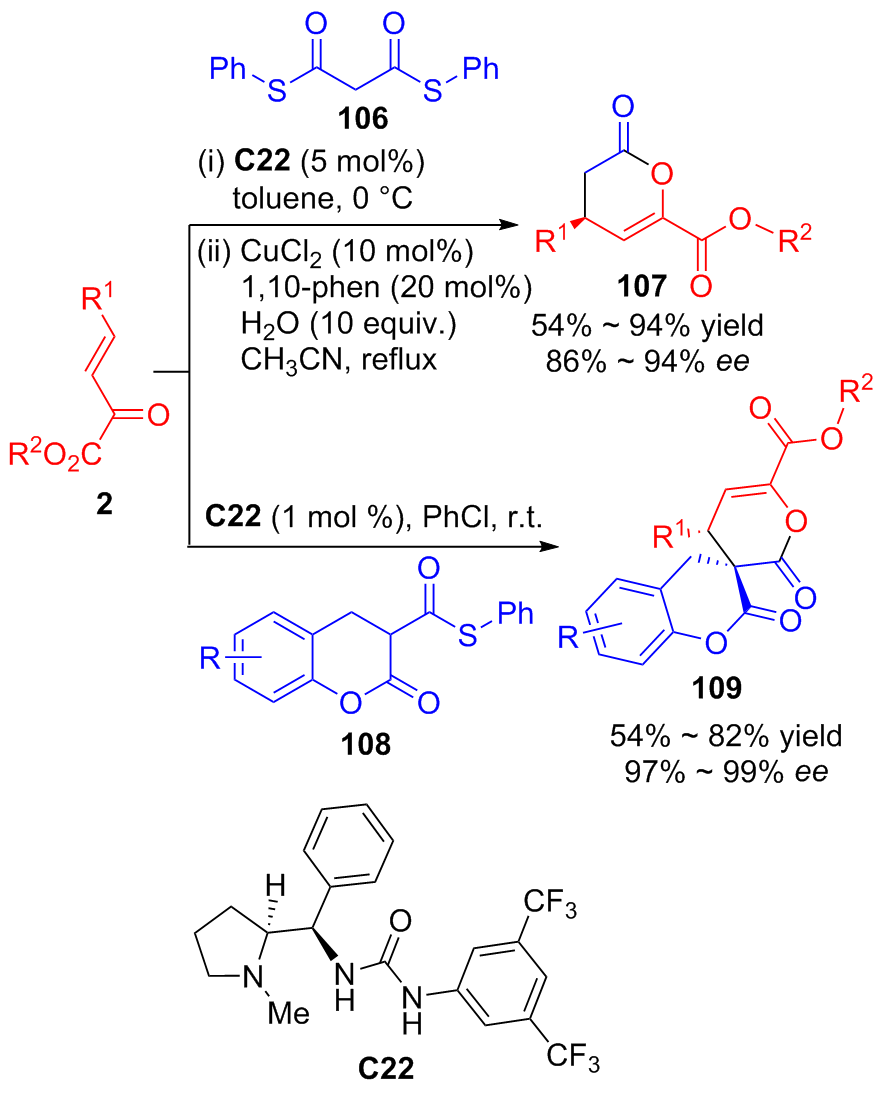


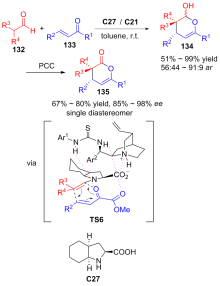


| [1] |
(a) Halliwell, B. Biochem. J. 2007, 401,1.
doi: 10.1042/BJ20061131 pmid: 17201405 |
|
(b) Robert, S.; Bertolla, C.; Masereel, B.; Dogné, J.-M.; Pochet, L. J. Med. Chem. 2008, 51,3077.
doi: 10.1021/jm8002697 pmid: 17201405 |
|
|
(c) Yang, J.; Liu, G.-Y.; Dai, F.; Cao, X.-Y.; Kang, Y.; Hu, L.-M.; Jin, X.-L. Bioorg. Med. Chem. Lett. 2011, 21,6420.
doi: 10.1016/j.bmcl.2011.08.090 pmid: 17201405 |
|
|
(d) Nantermet, P. G.; Barrow, J. C.; Selnick, H. G.; Homnick, C. F.; Freidinger, R. M.; Chang, R. S. L.; O’Malley, S. S.; Reiss, D. R.; Broten, T. P.; Ransom, R. W.; Pettibone, D. J.; Olah, T.; Forray, C. Bioorg. Med. Chem. Lett. 2000, 10,1625.
pmid: 17201405 |
|
|
(e) Goodman, K. B.; Cui, H.; Dowdell, S. E.; Gaitanopoulos, D. E.; Ivy, R. L.; Sehon, C. A.; Stavenger, R. A.; Wang, G. Z.; Viet, A. Q.; Xu, W.; Ye, G.; Semus, S. F.; Evans, C.; Fries, H. E.; Jolivette, L. J.; Kirkpatrick, R. B.; Dul, E.; Khandekar, S. S.; Yi, T.; Jung, D. K.; Wright, L. L.; Smith, G. K.; Behm, D. J.; Bentley, R.; Doe, C. P.; Hu, E.; Lee, D. J. Med. Chem. 2007, 50,6.
pmid: 17201405 |
|
| [2] |
(a) Ying, A.; Wu, C.; Fu, Y.; Ren, S.; Liang, H. Chin. J. Org. Chem. 2012, 32,1587(in Chinese).
doi: 10.6023/cjoc201203009 pmid: 32728081 |
|
( 应安国, 武承林, 付永前, 任世斌, 梁华定, 有机化学, 2012, 32,1587.)
doi: 10.6023/cjoc201203009 pmid: 32728081 |
|
|
(b) Xiang, S.-H.; Tan, B. Nat. Commun. 2020, 11,3786.
doi: 10.1038/s41467-020-17580-z pmid: 32728081 |
|
|
(c) Lassaletta, J. M. Nat. Commun. 2020, 11,3787.
doi: 10.1038/s41467-020-17600-y pmid: 32728081 |
|
|
(d) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76,838(in Chinese).
doi: 10.6023/A18060237 pmid: 32728081 |
|
|
( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76,838.)
pmid: 32728081 |
|
| [3] |
(a) Dalko, P. I. Comprehensive Enantioselective Organocata-lysis: Catalysts, Reactions, and Applications, Wiley, New York, 2013.
|
|
(b) Chen, X.-K.; Wang, H.-L.; Jin, Z.-C.; Chi, Y. R. Chin. J. Chem. 2020, 38,1167.
doi: 10.1002/cjoc.v38.10 |
|
| [4] |
(a) Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2,167.
doi: 10.1038/nchem.539 |
|
(b) Chanda, T.; Zhao, J. C. -G. Adv. Synth. Catal. 2018, 360,2.
doi: 10.1002/adsc.v360.1 |
|
|
(c) Lin, Y. ; D., D-M. Chin. J. Org. Chem. 2020, 40,3214(in Chinese).
doi: 10.6023/cjoc202005065 |
|
|
( 林晔, 杜大明, 有机化学, 2020, 40,3214.)
doi: 10.6023/cjoc202005065 |
|
|
(d) Sun, L.-H.; Liang, Z.-Q.; Ye, S. Acta Chim. Sinica 2014, 72,841(in Chinese).
doi: 10.6023/A14040334 |
|
|
( 孙利辉, 梁志钦, 叶松, 化学学报, 2014, 72,841.)
|
|
|
(e) Zheng, Y.; Xie, Z.-Z.; Chen, K.; Xiang, H.-Y.; Yang, H. Chin. J. Org. Chem. 2021, 41,1(in Chinese).
doi: 10.6023/cjoc202008037 |
|
|
( 郑雨, 谢珍珍, 陈凯, 向皞月, 阳华, 有机化学, 2021, 41,1.)
doi: 10.6023/cjoc202008037 |
|
| [5] |
Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M.; Smith, A. D. J. Am. Chem. Soc. 2011, 133,2714.
doi: 10.1021/ja109975c pmid: 21302961 |
| [6] |
(a) Oehlrich, D.; Berthelot, D. J. -C.; Gijsen, H. J. M. J. Med. Chem. 2011, 54,699.
doi: 10.1021/jm1010513 pmid: 22342629 |
|
(b) Chen, J.; Lu, M.-M.; Liu, B.; Chen, Z.; Li, Q.-B.; Tao, L.-J.; Hu, G.-Y. Bioorg. Med. Chem. Lett. 2012, 22,2300.
doi: 10.1016/j.bmcl.2012.01.073 pmid: 22342629 |
|
|
(c) Li, P.; Liu, L.-J.; Liu, J.-T. Org. Biomol. Chem. 2011, 9,74.
doi: 10.1039/C0OB00699H pmid: 22342629 |
|
|
(d) Yi, H.; Song, L. P.; Wang, W.; Liu, J. N.; Zhu, S. Z.; Deng, H. M.; Shao, M. Chem. Commun. 2010, 46,6941.
doi: 10.1039/c0cc01815e pmid: 22342629 |
|
| [7] |
Morrill, L. C.; Douglas, J.; Lebl, T.; Slawin, A. M.; Fox, D. J.; Smith, A. D. Chem. Sci. 2013, 4,4146.
doi: 10.1039/c3sc51791h |
| [8] |
Simal, C.; Lebl, T.; Slawin, A. M. Z.; Smith, A. D. Angew. Chem., Int. Ed. 2012, 51,3653.
doi: 10.1002/anie.v51.15 |
| [9] |
Yeh, P. P.; Daniels, D. S. B.; Fallan, C.; Gould, E.; Simal, C.; Taylor, J. E.; Smith, A. D. Org. Biomol. Chem. 2015, 13,2177.
doi: 10.1039/C4OB02408G |
| [10] |
Stark, D. G.; Young, C. M.; O’Riordan, T. J. C.; Slawin, A. M. Z.; Smith, A. D. Org. Biomol. Chem. 2016, 14,8068.
doi: 10.1039/C6OB01473A |
| [11] |
Stark, D. G.; Morrill, L. C.; Cordes, D. B.; Slawin, A. M. Z.; O’Riordan, T. J. C.; Smith, A. D. Chem. Asian J. 2016, 11,395.
doi: 10.1002/asia.v11.3 |
| [12] |
Young, C. M.; Stark, D. G.; West, T. H.; Taylor, J. E.; Smith, A. D. Angew. Chem., Int. Ed. 2016, 55,14394.
doi: 10.1002/anie.v55.46 |
| [13] |
Lee, J. W.; Mayer-Gall, T.; Opwis, K.; Song, C. E.; Gutmann, J. S.; List, B. Science 2013, 341,1225.
doi: 10.1126/science.1242196 |
| [14] |
Wang, S.; Izquierdo, J.; Rodríguez-Escrich, C.; Pericàs, M. A. ACS Catal. 2017, 7,2780.
doi: 10.1021/acscatal.7b00360 |
| [15] |
Zhang, Y.-C.; Geng, R.-L.; Song, J.; Gong, L.-Z. Org. Lett. 2020, 22,2261.
doi: 10.1021/acs.orglett.0c00461 |
| [16] |
Liu, H.; Slawin, A. M. Z.; Smith, A. D. Org. Lett. 2020, 22,1301.
doi: 10.1021/acs.orglett.9b04615 |
| [17] |
(a) Nozawa, K.; Nakajima, S. J. Nat. Prod. 1979, 42,374.
doi: 10.1021/np50004a004 |
|
(b) Koizumi, Y.; Arai, M.; Tomoda, H.; Omura, S. Biochim. Biophys. Acta, Mol. Cell Res. 2004,1693 , 47.
|
|
|
(c) He, R.; Ding, C.; Maruoka, K. Angew. Chem., Int. Ed. 2009, 48,4559.
doi: 10.1002/anie.v48:25 |
|
|
(d) Guo, C.; Song, J.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2010, 49,5558.
doi: 10.1002/anie.201002108 |
|
|
(e) Yao, J.; Wei, X.; Lu, Y. Biochem. Biophys. Res. Commun. 2016, 473,867.
doi: 10.1016/j.bbrc.2016.03.141 |
|
| [18] |
(a) Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115,9307.
doi: 10.1021/acs.chemrev.5b00060 pmid: 25992594 |
|
(b) Chen, X. -.; Gao, Z. -.; Ye, S. Acc. Chem. Res. 2020, 53,690.
doi: 10.1021/acs.accounts.9b00635 pmid: 25992594 |
|
|
(c) Wang, A.; Xiao, Y. -.; Zhou, Y.; Xu, J. -.; Liu, H. Chin. J. Org. Chem. 2017, 37,2590(in Chinese).
pmid: 25992594 |
|
|
( 王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37,2590.)
doi: 10.6023/cjoc201702041 pmid: 25992594 |
|
| [19] |
He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128,15088.
doi: 10.1021/ja066380r |
| [20] |
Wang, D.-L.; Liang, Z.-Q.; Chen, K.-Q.; Sun, D.-Q.; Ye, S. J. Org. Chem. 2015, 80,5900.
doi: 10.1021/acs.joc.5b00232 |
| [21] |
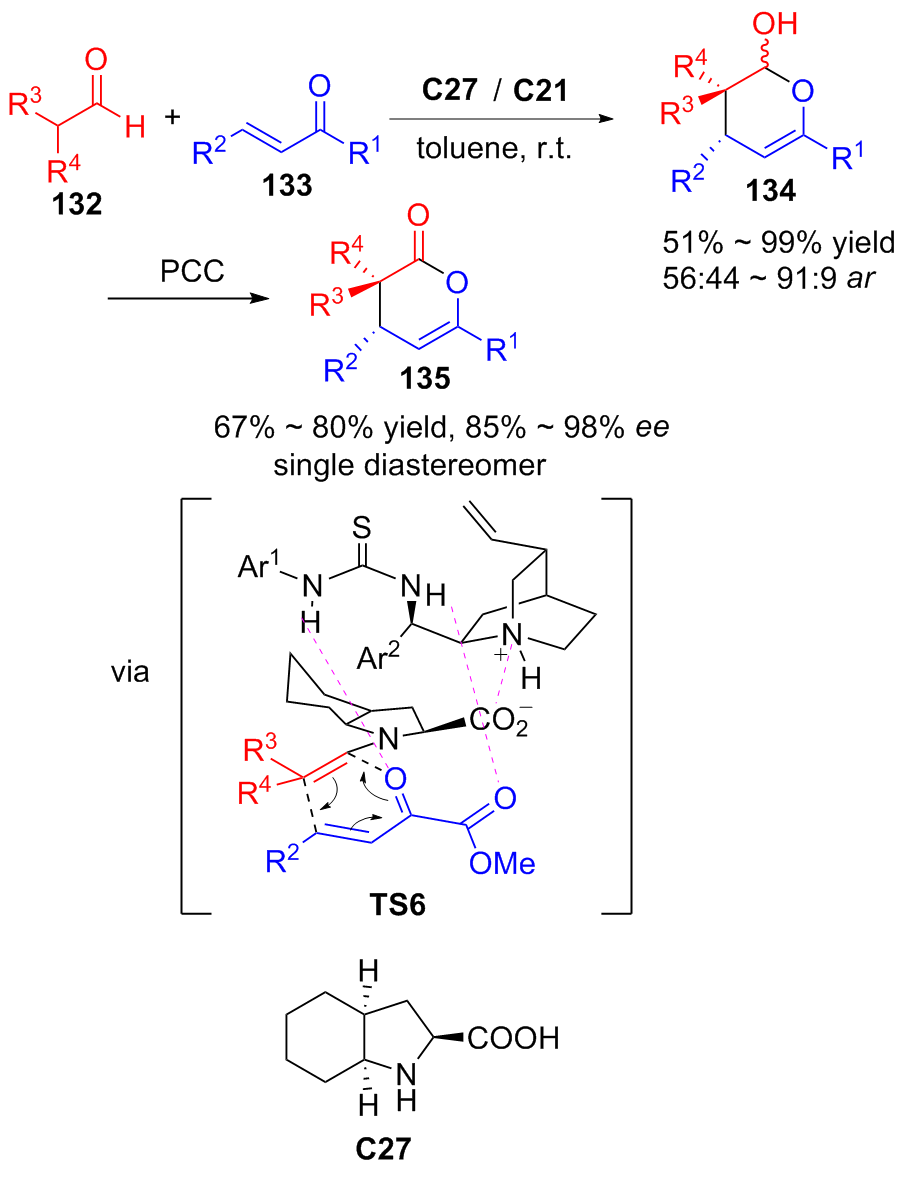
Davies, A. T.; Pickett, P. M.; Slawin, A. M. Z.; Smith, A. D. ACS Catal. 2014, 4,2696.
doi: 10.1021/cs500667g |
| [22] |
(a) Ulmschneider, S.; Muller-Vieira, U.; Klein, C. D.; Antes, I.; Lengauer, T.; Hartmann, R. W. J. Med. Chem. 2005, 48,1563.
pmid: 18667309 |
|
(b) Hudson, S.; Kiankarimi, M.; Eccles, W.; Mostofi, Y. S.; Genicot, M. J.; Dwight, W.; Fleck, B. A.; Gogas, K.; Wade, W. S. Bioorg. Med. Chem. Lett. 2008, 18,4495.
doi: 10.1016/j.bmcl.2008.07.050 pmid: 18667309 |
|
|
(c) Camps, P.; Formosa, X.; Galdeano, C.; Gomez, T.; Munoz-Tor- rero, D.; Scarpellini, M.; Viayna, E.; Badia, A.; Clos, M. V.; Camins, A.; Pallas, M.; Bartolini, M.; Mancini, F.; Andrisano, V.; Estelrich, J.; Lizondo, M.; Bidon-Chanal, A.; Luque, F. J. J. Med. Chem. 2008, 51,3588.
doi: 10.1021/jm8001313 pmid: 18667309 |
|
| [23] |
Biswas, A.; Sarkar, S. D.; Fröhlich, R.; Studer, A. Org. Lett. 2011, 13,4966.
doi: 10.1021/ol202108a pmid: 21863845 |
| [24] |
Mukherjee, S.; Ghosh, A.; Marelli, U. K.; Biju, A. T. Org. Lett. 2018, 20,2952.
doi: 10.1021/acs.orglett.8b00998 pmid: 29722983 |
| [25] |
Hao, L.; Chen, S.; Xu, J.; Tiwari, B.; Fu, Z., Li, T.; Chi, Y. R. Org. Lett. 2013, 15,4956.
doi: 10.1021/ol4021805 |
| [26] |
Gharpure, S. J.; Vishwakarma, D. S. Eur. J. Org. Chem. 2020,6887.
|
| [27] |
Lee, A.; Younai, A.; Price, C. K.; Izquierdo, J.; Mishra, R. K.; Scheidt, K. A. J. Am. Chem. Soc. 2014, 136,10589.
doi: 10.1021/ja505880r |
| [28] |
(a) Lv, H.; Zhang, Y.-R.; Huang, X.-L.; Ye, S. Adv. Synth. Catal. 2008, 350,2715.
doi: 10.1002/adsc.200800532 |
|
(b) Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. Angew. Chem., Int. Ed. 2009, 48,192.
|
|
|
(c) Shao, P.-L.; Chen, X.-Y.; Ye, S. Angew. Chem., Int. Ed. 2010, 49,8412.
doi: 10.1002/anie.201003532 |
|
|
(d) Shao, P.-L.; Chen, X.-Y.; Sun, L.-H.; Ye, S. Tetrahedron Lett. 2010, 51,2316.
doi: 10.1016/j.tetlet.2010.02.122 |
|
|
(e) Zhang, H.-M.; Gao, Z.-H.; Ye, S. Org. Lett. 2014, 16,3079.
doi: 10.1021/ol501205v |
|
| [29] |
Lv, H.; Chen, X.-Y.; Sun, L.; Ye, S. J. Org. Chem. 2010, 75,6973.
doi: 10.1021/jo101318u |
| [30] |
Jian, T.-Y.; Chen, X.-Y.; Sun, L.-H.; Ye, S. Org. Biomol. Chem. 2013, 11,158.
doi: 10.1039/C2OB26804C |
| [31] |
Wang, C.-Y.; Wang, Z.-Y.; Yang, J.; Shi, S.-H.; Hui, X.-P. Org. Lett. 2020, 22,4440.
doi: 10.1021/acs.orglett.0c01447 |
| [32] |
(a) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132,8810.
doi: 10.1021/ja103631u pmid: 20550127 |
|
(b) Ryan, S. J.; Candish, L.; Lupton, D. W. J. Am. Chem. Soc. 2009, 131,14176.
doi: 10.1021/ja905501z pmid: 20550127 |
|
| [33] |
(a) De, Sarkar, S.; Studer, A. Angew. Chem., Int. Ed. 2010, 49,9266.
doi: 10.1002/anie.201004593 |
|
(b) Rong, Z.-Q.; Jia, M.-Q.; You, S.-L. Org. Lett. 2011, 13,4080.
doi: 10.1021/ol201595f |
|
| [34] |
Kravina, A. G.; Mahatthananchai, J.; Bode, J. W. Angew. Chem., Int. Ed. 2012, 51,9433.
doi: 10.1002/anie.201204145 |
| [35] |
(a) Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10,5165.
doi: 10.1039/c2ob25184a pmid: 22581310 |
|
(b) Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, III, C. F. ACS. Catal. 2014, 4,743.
doi: 10.1021/cs401172r pmid: 22581310 |
|
|
(c) Pavlovska, T. L.; Redkin, R. G.; Lipson, V. V.; Atamanuk, D. V. Mol. Diversity 2016, 20,299.
doi: 10.1007/s11030-015-9629-8 pmid: 22581310 |
|
|
(d) Yu, B.; Cai, Z.; Wang, S.; Liu, H. Chin. J. Org. Chem. 2017, 37,1952(in Chinese).
doi: 10.6023/cjoc201704004 pmid: 22581310 |
|
|
( 余斌, 蔡祖恽, 王帅, 刘宏民, 有机化学, 2017, 37,1952.)
doi: 10.6023/cjoc201704004 pmid: 22581310 |
|
| [36] |
Xie, D.; Yang, L.; Lin, Y.; Zhang, Z.; Chen, D.; Zeng, X.; Zhong, G. Org. Lett. 2015, 17,2318.
doi: 10.1021/acs.orglett.5b00726 |
| [37] |
Zhao, L.-L.; Li, X.-S., Cao, L.-L.; Zhang, R.; Shi, X.-Q.; Qi, J. Chem. Commun. 2017, 53,5985.
doi: 10.1039/C7CC02753B |
| [38] |
Piera, J.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2008, 47,3506.
doi: 10.1002/(ISSN)1521-3773 |
| [39] |
Xie, D.; Shen, D.; Chen, Q.; Zhou, J.; Zeng, X.; Zhong, G. J. Org. Chem. 2016, 81,6136.
doi: 10.1021/acs.joc.6b01152 |
| [40] |
Axelsson, A.; Hammarvid, E.; Ta, L.; Sundén, H. Chem. Commun. 2016, 52,11571.
doi: 10.1039/C6CC06060A |
| [41] |
Sun, F.-G.; Sun, L.-H.; Ye, S. Adv. Synth. Catal. 2011, 353,3134.
doi: 10.1002/adsc.v353.17 |
| [42] |
Zhang, H.; Jia, W.; Liang, Z.; Ye, S. Asian J. Org. Chem. 2014, 3,462.
doi: 10.1002/ajoc.v3.4 |
| [43] |
Gao, Z.-H.; Chen, X.-Y.; Zhang, H.-M.; Ye, S. Chem. Commun. 2015, 51,12040.
doi: 10.1039/C5CC04593B |
| [44] |
Chen, K.; Gao, Z.; Ye, S. Angew. Chem., Int. Ed. 2019, 58,1183.
doi: 10.1002/anie.v58.4 |
| [45] |
Yan, J.; Song, Z.; Zhao, C.; Shi, K.; Yang, L.; Zhong, G. Org. Lett. 2020, 22,3329.
doi: 10.1021/acs.orglett.0c00699 |
| [46] |
Cheng, J.; Huang, Z.; Chi, Y. R. Angew. Chem., Int. Ed. 2013, 52,8592.
doi: 10.1002/anie.201303247 |
| [47] |
Zhang, Z.; Zeng, X.; Xie, D.; Chen, D.; Ding, L.; Wang, A.; Zhong, G. Org. Lett. 2015, 17,5052.
doi: 10.1021/acs.orglett.5b02527 |
| [48] |
Chen, X.-Y.; Gao, Z.-H.; Song, C.-Y.; Zhang, C.-L.; Wang, Z.-X.; Ye, S. Angew. Chem., Int. Ed. 2014, 53,11611.
doi: 10.1002/anie.201407469 |
| [49] |
Que, Y.; Lu, Y.; Wang, W.; Wang, Y.; Wang, H.; Yu, C.; Yao, C. Chem. Asian J. 2016, 11,678.
doi: 10.1002/asia.201501353 |
| [50] |
(a) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125,12672.
doi: 10.1021/ja036972z |
|
(b) Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. J. Am. Chem. Soc. 2005, 127,119.
doi: 10.1021/ja044370p |
|
|
(c) Connon, S. J. Chem Commun. 2008, 22,2499.
|
|
| [51] |
Zhang, M. L.; Wu, Z. J.; Zhao, J. Q.; Luo, Y.; Xu, X. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2016, 18,5110.
doi: 10.1021/acs.orglett.6b02558 |
| [52] |
(a) Burke, H. M.; McSweeney, L.; Scanlan, E. M. Nat. Commun. 2017, 8,15655.
doi: 10.1038/ncomms15655 pmid: 27340449 |
|
(b) Bahlinger, A.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2014, 53,8779.
doi: 10.1002/anie.201310532 pmid: 27340449 |
|
|
(c) Schäberle, T. F. Beilstein J. Org. Chem. 2016, 12,571.
doi: 10.3762/bjoc.12.56 pmid: 27340449 |
|
| [53] |
Jin, H.; Lee, J.; Shi, H.; Lee, J. Y.; Yoo, E. J.; Song, C. E.; Ryu, D. H. Org. Lett. 2018, 20,1584.
doi: 10.1021/acs.orglett.8b00331 |
| [54] |
Cui, L. Y.; Lv, D.; Wang, Y. M.; Fan, Z. J.; Li, Z. M.; Zhou, Z. H. J. Org. Chem. 2018, 83,4221.
doi: 10.1021/acs.joc.8b00234 |
| [55] |
Zhang, S.; Lv, M.; Yin, S.; Li, N.; Zhang, J.; Wang, X. Adv. Synth. Catal. 2016, 358,143.
doi: 10.1002/adsc.v358.1 |
| [56] |
Zhang, Z.-P.; Chen, L.; Li, X.; Cheng, J.-P. J. Org. Chem. 2018, 83,2714.
doi: 10.1021/acs.joc.7b03177 |
| [57] |
Xian, J.; Chen, L.; Ye, L.; Sun, Y.; Shi, Z.; Zhao, Z.; Li, X. Tetrahedron 2019, 75,2350.
doi: 10.1016/j.tet.2019.03.007 |
| [58] |
Chen, L.; Zhang, X.; Shi, K.-J.; Leng, H.-J.; Li, Q.-Z.; Liu, Y.; Li, J.-L. J. Org. Chem. 2020, 85,9454.
doi: 10.1021/acs.joc.0c00957 |
| [59] |
(a) GrAger, H.; Wilken, J. Angew. Chem., Int. Ed. 2001, 40,529.
doi: 10.1002/1521-3773(20010202)40:3【-逻*辑*与-】#x00026;lt;【-逻*辑*与-】#x00026;gt;1.0.CO;2-A |
|
(b) List, B. Tetrahedron 2002, 58,5573.
doi: 10.1016/S0040-4020(02)00516-1 |
|
|
(c) Marigo, M.; Wabnitz, T. C.; Fielenbach, D.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44,794.
doi: 10.1002/anie.v44:5 |
|
|
(d) Hayashi, Y.; Gotoh, H.; Hayashi, T.; Shoji, M. Angew. Chem., Int. Ed. 2005, 44,4212.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(e) Zhao, C.; Feng, Z.; Xu, G.; Gao, A.; Chen, J.; Wang, Z.; Xu, P. Angew. Chem., Int. Ed. 2020, 59,3058.
doi: 10.1002/anie.v59.8 |
|
| [60] |
Juhl, K.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2003, 42,1498.
doi: 10.1002/anie.200250652 |
| [61] |
Wang, J.; Yu, F.; Zhang, X.; Ma, D. Org. Lett. 2008. 10,2561.
doi: 10.1021/ol800835m |
| [62] |
Sinha, D.; Perera, S.; Zhao, J. C. G. Chem.-Eur. J. 2013, 19,6976.
doi: 10.1002/chem.v19.22 |
| [63] |
Kano, T.; Maruyama, H.; Homma, C.; Maruoka, K. Chem. Commun. 2018, 54,3496.
doi: 10.1039/C8CC01443D |
| [64] |
Kelley, A. M.; Haywood, R. D.; White, J. C.; Petersen, K. S. ChemistrySelect 2020, 5,3018.
doi: 10.1002/slct.v5.10 |
| [65] |
Shirakawa, S.; Maruoka, K. Angew. Chem. Int. Ed. 2013, 52,4312.
doi: 10.1002/anie.201206835 |
| [66] |
Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D. C. M.; Bencivenni, G.; Gillick-Healy, M. W.; Adamo, M. F. A. J. Org. Chem. 2020, 85,5183.
doi: 10.1021/acs.joc.9b03216 |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [6] | 全翌雯, 蒋心惠, 李文军, 汪舰. 借助有机催化去共轭-羟醛缩合反应来获得α-乙烯基-β-炔基取代的烯醛[J]. 有机化学, 2023, 43(6): 2120-2125. |
| [7] | 景科, 张攀科, 徐森苗. 1,4-氮硼杂芳环在有机和过渡金属催化中的应用[J]. 有机化学, 2023, 43(5): 1742-1750. |
| [8] | 张心予, 耿慧慧, 张士磊, 王卫, 陈晓蓓. 一种N-杂环卡宾催化合成氘代苯偶姻的方法[J]. 有机化学, 2023, 43(4): 1510-1516. |
| [9] | 戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090. |
| [10] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [11] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [12] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [13] | 张雨杉, 桓臻, 杨金东, 程津培. 氮杂环磷氢试剂的氢转移活性研究进展[J]. 有机化学, 2023, 43(11): 3806-3825. |
| [14] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [15] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||