有机化学 ›› 2023, Vol. 43 ›› Issue (3): 1084-1090.DOI: 10.6023/cjoc202301011 上一篇 下一篇
所属专题: 中国女科学家专辑
研究论文
收稿日期:2023-01-10
修回日期:2023-02-20
发布日期:2023-02-27
通讯作者:
杨丽敏
作者简介:基金资助:
Chunbo Dai, Siqi Xia, Xiaoyu Chen, Limin Yang( )
)
Received:2023-01-10
Revised:2023-02-20
Published:2023-02-27
Contact:
Limin Yang
About author:Supported by:文章分享

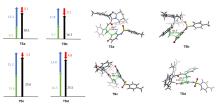
报道了4-氨基苯并环庚烯内酯的合成方法, 在氮杂环卡宾的催化作用下, γ-氯醛与亚胺醌化合物经过[4+3]环加成反应生成4-氨基苯并环庚烯内酯, 产率良好, 且反应产物单一, 反应条件温和, 操作简便, 符合绿色化学原则. 为了探究氮杂环卡宾在该反应的位点选择性所起的作用, 利用密度泛函理论进行了理论研究, 研究表明形变力是导致位点选择差异性的主要原因.
戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090.
Chunbo Dai, Siqi Xia, Xiaoyu Chen, Limin Yang. N-Heterocyclic Carbene (NHC)-Catalyzed [4+3] Cycloaddition to Synthesize 4-Aminobenzoheptenolactons[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1084-1090.
| Entry | Solvent | Cat. | Base | T/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | THF | Cat. A | TEA | r.t | 30 |
| 2 | THF | Cat. B | TEA | r.t | 35 |
| 3 | THF | Cat. C | TEA | r.t | <10 |
| 4 | THF | Cat. D | TEA | r.t | Trace |
| 5 | THF | Cat. E | TEA | r.t | Trace |
| 6 | THF | Cat. F | TEA | r.t | Trace |
| 7 | THF | Cat. G | TEA | r.t | Trace |
| 8 | THF | Cat. B | DIPEA | r.t | Trace |
| 9 | THF | Cat. B | DABCO | r.t | Trace |
| 10 | THF | Cat. B | DMAP | r.t | Trace |
| 11 | THF | Cat. B | DBU | r.t | Trace |
| 12 | THF | Cat. B | Na2CO3 | r.t | 20 |
| 13 | THF | Cat. B | NaOAc | r.t | 40 |
| 14 | THF | Cat. B | Cs2CO3 | r.t | 22 |
| 15 | THF | Cat. B | NaHCO3 | r.t | <10 |
| 16 | THF | Cat. B | K2CO3 | r.t | <10 |
| 17 | DCM | Cat. B | NaOAc | r.t | 65 |
| 18 | DCE | Cat. B | NaOAc | r.t | 52 |
| 19 | CHCl3 | Cat. B | NaOAc | r.t | 56 |
| 20 | Et2O | Cat. B | NaOAc | r.t | 64 |
| 21 | Toluene | Cat. B | NaOAc | r.t | 74 |
| 22 | 1,4-Dioxane | Cat. B | NaOAc | r.t | Trace |
| 23 | EA | Cat. B | NaOAc | r.t | 50 |
| 24 | MTBE | Cat. B | NaOAc | r.t | <10 |
| 25 | MeCN | Cat. B | NaOAc | r.t | <10 |
| 26 | Toluene | Cat. B | NaOAc | 0 | 30 |
| 27 | Toluene | Cat. B | NaOAc | 30 | 70 |
| 28c | Toluene | Cat. B | NaOAc | r.t | 51 |
| 29d | Toluene | Cat. B | NaOAc | r.t | 70 |
| Entry | Solvent | Cat. | Base | T/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | THF | Cat. A | TEA | r.t | 30 |
| 2 | THF | Cat. B | TEA | r.t | 35 |
| 3 | THF | Cat. C | TEA | r.t | <10 |
| 4 | THF | Cat. D | TEA | r.t | Trace |
| 5 | THF | Cat. E | TEA | r.t | Trace |
| 6 | THF | Cat. F | TEA | r.t | Trace |
| 7 | THF | Cat. G | TEA | r.t | Trace |
| 8 | THF | Cat. B | DIPEA | r.t | Trace |
| 9 | THF | Cat. B | DABCO | r.t | Trace |
| 10 | THF | Cat. B | DMAP | r.t | Trace |
| 11 | THF | Cat. B | DBU | r.t | Trace |
| 12 | THF | Cat. B | Na2CO3 | r.t | 20 |
| 13 | THF | Cat. B | NaOAc | r.t | 40 |
| 14 | THF | Cat. B | Cs2CO3 | r.t | 22 |
| 15 | THF | Cat. B | NaHCO3 | r.t | <10 |
| 16 | THF | Cat. B | K2CO3 | r.t | <10 |
| 17 | DCM | Cat. B | NaOAc | r.t | 65 |
| 18 | DCE | Cat. B | NaOAc | r.t | 52 |
| 19 | CHCl3 | Cat. B | NaOAc | r.t | 56 |
| 20 | Et2O | Cat. B | NaOAc | r.t | 64 |
| 21 | Toluene | Cat. B | NaOAc | r.t | 74 |
| 22 | 1,4-Dioxane | Cat. B | NaOAc | r.t | Trace |
| 23 | EA | Cat. B | NaOAc | r.t | 50 |
| 24 | MTBE | Cat. B | NaOAc | r.t | <10 |
| 25 | MeCN | Cat. B | NaOAc | r.t | <10 |
| 26 | Toluene | Cat. B | NaOAc | 0 | 30 |
| 27 | Toluene | Cat. B | NaOAc | 30 | 70 |
| 28c | Toluene | Cat. B | NaOAc | r.t | 51 |
| 29d | Toluene | Cat. B | NaOAc | r.t | 70 |

| [1] |
(a) Yu, Y.; Pushkin, C.; Song, J.; Zhu, L.; Li, C.; Huang, X. Nat. Commun. 2020, 11, 461.
doi: 10.1038/s41467-019-14101-5 |
|
(b) Fan, X.-D.; Wang, G.-W.; Wang, H.; Mao, J.-X.; Lan, X.-Z.; Miao, Z.-H.; Chen, M. Chin. Acta Pharm Sin. 2016, 51, 770. (in Chinese)
|
|
|
(范旭东, 王国伟, 王红, 毛景欣, 兰小中, 廖志华, 陈敏, 药学学报, 2016, 51, 770.)
|
|
| [2] |
(a) Schleyer, P. V. R.; Williams, J. E.; Blanchard, K. R. J. Am. Chem. Soc. 1970, 92, 2377.
doi: 10.1021/ja00711a030 |
|
(b) Xu, H.; Hu, J.; Wang, L.; Liao, S.; Tang, Y. J. Am. Chem. Soc. 2015, 137, 8006.
doi: 10.1021/jacs.5b04429 |
|
|
(c) Hu, Y.; Li, L.; Han, J.; Min, L.; Li, C. Chem. Rev. 2020, 120, 5910.
doi: 10.1021/acs.chemrev.0c00045 |
|
| [3] |
(a) Rezai, T.; Yu, B.; Millhauser, G. L.; Jacobson, M. P.; Lokey, R. J. Am. Chem. Soc. 2006, 128, 2510.
pmid: 16492015 |
|
(b) Hung, K.; Hu, X.; Maimone, T. J. Nat. Prod. Rep. 2018, 35, 174.
doi: 10.1039/C7NP00065K pmid: 16492015 |
|
|
(c) Wang, L.-N.; Yu, Z.-X. Chin. J. Org. Chem. 2020, 40, 3536. (in Chinese)
doi: 10.6023/cjoc202010025 pmid: 16492015 |
|
|
(王路宁, 余志祥, 有机化学, 2020, 40, 3536.)
doi: 10.6023/cjoc202010025 pmid: 16492015 |
|
|
(d) Reyes, R. L.; Iwai, T.; Sawamura, M. Chem. Rev. 2021, 121, 8926.
doi: 10.1021/acs.chemrev.0c00793 pmid: 16492015 |
|
| [4] |
Illuminati, G.; Mandolini, L. Acc. Chem. Res. 1981, 14, 95.
doi: 10.1021/ar00064a001 |
| [5] |
(a) Butenschon, H. Angew. Chem., Int. Ed. 2008, 47, 5287
doi: 10.1002/anie.v47:29 pmid: 33576620 |
|
(b) Yet, L. Chem. Rev. 2000, 100, 2963.
pmid: 33576620 |
|
|
(c) Doerksen, R.S.; Hodik, T.; Hu, G.; Huynh, N. O.; Shuler, Q. W. G.; Krische, M. J. Chem. Rev. 2021, 121, 4045.
doi: 10.1021/acs.chemrev.0c01133 pmid: 33576620 |
|
| [6] |
(a) Meng, D.; Xie, Y.; Peng, Q.; Wang, J. Org. Lett. 2020, 22, 7635.
doi: 10.1021/acs.orglett.0c02832 pmid: 32964708 |
|
(b) Wang, J.; Zhao, C.; Wang, J. ACS Catal. 2021, 11, 12520.
doi: 10.1021/acscatal.1c03459 pmid: 32964708 |
|
|
(c) Gao, J.; Feng, J.; Du, D. Org. Chem. Front. 2021, 8, 6138.
doi: 10.1039/D1QO01127H pmid: 32964708 |
|
|
(d) Pavithra, T.; Devi, E.; Maheswari, C. Asian J. Org. Chem. 2021, 10, 1861.
doi: 10.1002/ajoc.v10.8 pmid: 32964708 |
|
| [7] |
Burstein, C.; Glorius, F. Angew. Chem., Int. Ed. 2004, 43, 6205.
doi: 10.1002/(ISSN)1521-3773 |
| [8] |
Sohn, S.; Rosen, E.; Bode, J. J. Am. Chem. Soc. 2004, 126, 14370.
doi: 10.1021/ja044714b |
| [9] |
(a) Ghosh, A.; Barik, S.; Biju. A. Org. Lett. 2019, 21, 8598.
doi: 10.1021/acs.orglett.9b03188 pmid: 32312044 |
|
(b) Dzieszkowski, K.; Baranska. I.; Rafinski. Z. J. Org. Chem. 2020, 85, 6645.
doi: 10.1021/acs.joc.0c00657 pmid: 32312044 |
|
|
(c) Duan, X.; Tian, Z.; Liu, B.; He, T.; Zhao, L.; Dong, M.; Zhang, P.; Qi, J. Org. Lett. 2021, 23, 3777.
doi: 10.1021/acs.orglett.1c01203 pmid: 32312044 |
|
| [10] |
(a) Xie, D.; Yang, L.; Lin, Y.; Zhang, Z.; Chen, D.; Zeng, X.; Zhong, G. Org. Lett. 2015, 17, 2318.
doi: 10.1021/acs.orglett.5b00726 |
|
(b) Lin, Y.; Yang, L.; Deng, Y.; Zhong, G. Chem. Commun. 2015, 51, 8330.
doi: 10.1039/C5CC02096D |
|
| [11] |
(a) Zhang, Y.; Lv, H.; Zhou, D.; Ye, S. Chem.-Eur. J. 2008, 14, 8473.
doi: 10.1002/chem.v14:28 |
|
(b) Lv, H.; You, L.; Ye, S. Adv. Synth. Catal. 2009, 351, 2822.
doi: 10.1002/adsc.v351:17 |
|
|
(c) Huang, X.; He, L.; Shao, P.; Ye, S. Angew. Chem., Int. Ed. 2009, 48, 192.
|
|
|
(d) Mo, J.; Chen, X.; Chi, Y. J. Am. Chem. Soc. 2012, 134, 8810.
doi: 10.1021/ja303618z |
|
|
(e) Yao, C.; Xiao, Z.; Liu, R.; Li, T.; Jiao, W.; Yu, C. Chem.-Eur. J. 2013, 19, 456.
doi: 10.1002/chem.201202655 |
|
| [12] |
Yan, J.; Song, Z.; Zhao, C.; Shi, K.; Yang, L.; Zhong, G. Org. Lett. 2020, 22, 9, 3329.
|
| [13] |
Lv, H.; Jia, W.; Sun, L.; Ye, S. Angew. Chem., Int. Ed. 2013, 52, 8607.
doi: 10.1002/anie.201303903 |
| [14] |
Izquierdo, J.; Orue, A.; Scheidt, K. A. J. Am. Chem. Soc. 2013, 135, 10634.
doi: 10.1021/ja405833m pmid: 23829462 |
| [1] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [2] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [3] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [4] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [5] | 杨亮茹, 郭梦丽, 袁金伟, 王佳美, 夏宇婷, 肖咏梅, 毛璞. 钳形氮杂环卡宾金属络合物的研究进展[J]. 有机化学, 2023, 43(6): 2002-2025. |
| [6] | 全翌雯, 蒋心惠, 李文军, 汪舰. 借助有机催化去共轭-羟醛缩合反应来获得α-乙烯基-β-炔基取代的烯醛[J]. 有机化学, 2023, 43(6): 2120-2125. |
| [7] | 景科, 张攀科, 徐森苗. 1,4-氮硼杂芳环在有机和过渡金属催化中的应用[J]. 有机化学, 2023, 43(5): 1742-1750. |
| [8] | 张心予, 耿慧慧, 张士磊, 王卫, 陈晓蓓. 一种N-杂环卡宾催化合成氘代苯偶姻的方法[J]. 有机化学, 2023, 43(4): 1510-1516. |
| [9] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [10] | 张雨杉, 桓臻, 杨金东, 程津培. 氮杂环磷氢试剂的氢转移活性研究进展[J]. 有机化学, 2023, 43(11): 3806-3825. |
| [11] | 赵薇, 刘京, 何向奎, 蒋豪, 陆良秋, 肖文精. 氮杂环卡宾(NHC)催化的联芳基二醛去对称化构建轴手性醛类化合物[J]. 有机化学, 2022, 42(8): 2504-2514. |
| [12] | 王兴, 宋倩倩, 陈续玲, 李鹏飞, 齐昀坤, 李文军. 有机催化远程立体控制6-亚甲基-6H-吲哚与异噁唑-5(4H)-酮的氮杂1,8-共轭加成反应[J]. 有机化学, 2022, 42(6): 1722-1734. |
| [13] | 马志伟, 陈晓培, 王川川, 王建玲, 陶京朝, 吕全建. 手性方酰胺催化环状1,3-二羰基化合物对β,γ-不饱和-α-酮酯的不对称Michael加成反应[J]. 有机化学, 2022, 42(5): 1520-1526. |
| [14] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| [15] | 姚婷, 李佳燕, 王佳明, 赵常贵. 氮杂环卡宾催化构筑含七元环结构的研究进展[J]. 有机化学, 2022, 42(4): 925-944. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||